Apple has redesigned its Apple Watch Series 9 and Apple Watch Ultra 2 models to “not contain pulse oximetry functionality,” medical technology company Masimo said in a document filed today with the U.S. Court of Appeals for the Federal Circuit.
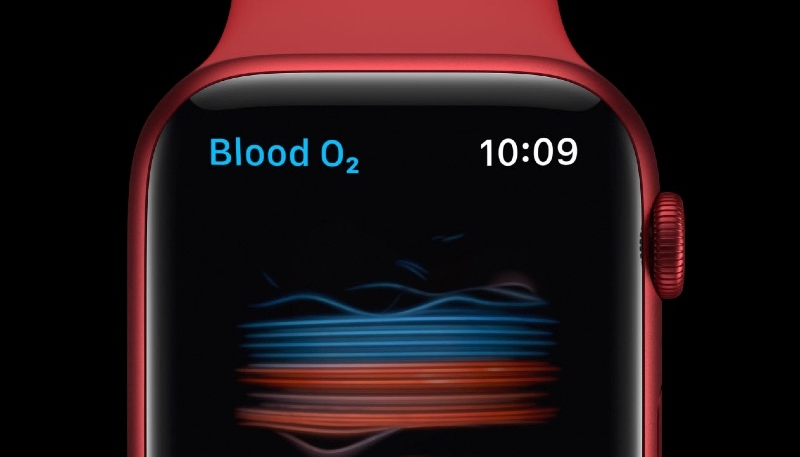
The filing, first shared by MacRumors, suggests that Apple will be removing the Blood Oxygen app from new Apple Watch Series 9 and Apple Watch Ultra 2 models sold in the U.S., at least temporarily.
By removing the app, Apple could sidestep an import and sales ban on Apple Watch models with blood oxygen sensing, which the U.S. International Trade Commission ordered last year, following a ruling that Apple violated Masimo’s pulse oximetry patents.
Apple was able to get a temporary pause of the ban shortly after it began last month, but it could have resumed as early as this month.
Bloomberg‘s Mark Gurman says Apple has started shipping modified Apple Watch models to retail stores in the U.S., but it is unclear when they will go on sale.
The plan was disclosed Monday by Masimo Corp., which has been locked in a feud with Apple over patents related to the technology. It said that US Customs and Border Protection approved the move on Jan. 12. The agency “decided that Apple’s redesign falls outside the scope” of an import ban by the US International Trade Commission, signaling that the adjustment will let Apple keep its watches on the market.
Apple has not yet made an official comment about the modified Watches.
Apple’s website still advertises the Blood Oxygen app as an available feature on the Series 9 and Ultra 2 order pages in the U.S. as of writing. Existing owners of the Series 9 and Ultra 2 will likely retain access to the feature, which is not expected to be removed from models sold in other countries around the globe.
Apple is likely making the move as a short-term solution, as Apple is reportedly working on a software update that would avoid Masimo’s patented blood oxygen measuring technology.