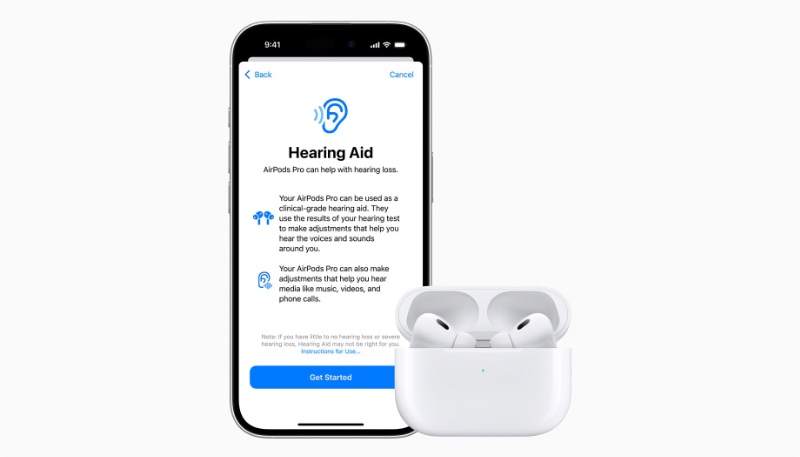
Apple’s AirPods Pro 2 earbuds will soon be able to act as over-the-counter hearing Aids, and the company has now received approval for the feature from the United States Food and Drug Administration (FDA)
As first noted by TechCrunch, the FDA on Thursday announced that it has authorized Apple to add OTC hearing aid functionality to the AirPods Pro 2, making it the first product that the FDA has allowed to serve as an over-the-counter hearing aid software device.
Later this year, AirPods Pro 2 feature list will include what Apple calls the world’s first end-to-end hearing health experience. New features will include active Hearing Protection, a hearing test, and a hearing aid feature. After taking the hearing test with their AirPods Pro 2 earbuds and iPhone, users will be able to view a summary of their test results, which is stored privately and securely in the Health app. Users can also share test results with their healthcare providers.
if hearing loss is detected, a personalized sound profile is created that allows the AirPods Pro 2 to make dynamic sound adjustments to increase sound as needed.
Apple says that the clinical-grade hearing aid feature will help people better engage in conversation and stay connected to the people and environment around them. Personalized hearing profiles are then automatically applied to music, movies, games, and phone calls without the need to adjust any settings.
Both the Hearing Test and Hearing Aid features are soon expected to be given marketing consent from global health authorities, and both tests will be available this fall in 100+ countries and regions, including the United States, Germany, and Japan.
The new hearing health features will be available this fall for AirPods Pro 2 customers in a free software update when paired with iPhone or iPad running iOS 18 or iPadOS 18.